Description
Product information
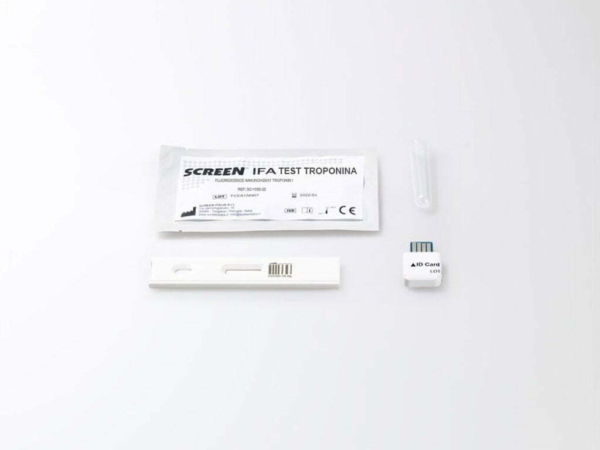
IFA Troponin I Test (Whole Blood/Serum/Plasma) is a rapid, quantitative diagnostic test for the detection of cardiac Troponin I (cTnI), a key biomarker for myocardial infarction (MI). Utilizing the Immunofluorescence Analyzer, this test is intended for professional in vitro diagnostic (IVD) use and delivers reliable results from whole blood, serum, or plasma samples. Designed for professional in vitro diagnostic use only.
Why Measure Cardiac Troponin I (cTnI)?
- Diagnosing acute myocardial infarction (heart attack)
- Detecting heart muscle damage in emergency or perioperative settings
- Monitoring ischemic heart conditions, such as unstable angina or congestive heart failure
- Evaluating cardiac complications after surgery or trauma
Compared to other biomarkers like CK-MB, cTnI remains elevated for 6–10 days, providing a longer detection window for heart injury.
How It Works
The sample (whole blood, serum, or plasma) flows across the test strip.
- If cTnI is present, it binds to fluorescently labeled anti-cTnI antibodies.
- This immune complex is captured by additional anti-cTnI antibodies immobilized on the nitrocellulose membrane (test line).
- The Immunofluorescence Analyzer measures the fluorescence intensity, which correlates with the cTnI concentration in the sample.
Key Features:
- Rapid, quantitative results
- High sensitivity and specificity
- Easy-to-use format suitable for point-of-care and clinical use
Clinical Significance
- High tissue specificity
- Early release (4–6 hours post-injury)
- Prolonged elevation compared to CK-MB
Additional Info
Additional information
| Product name | IFA Troponin I |
|---|---|
| Detection | Cardiac Troponin I (cTnI) |
| Sample Type | |
| Pack Size | |
| Format | |
| Analyte Detection |