Categories
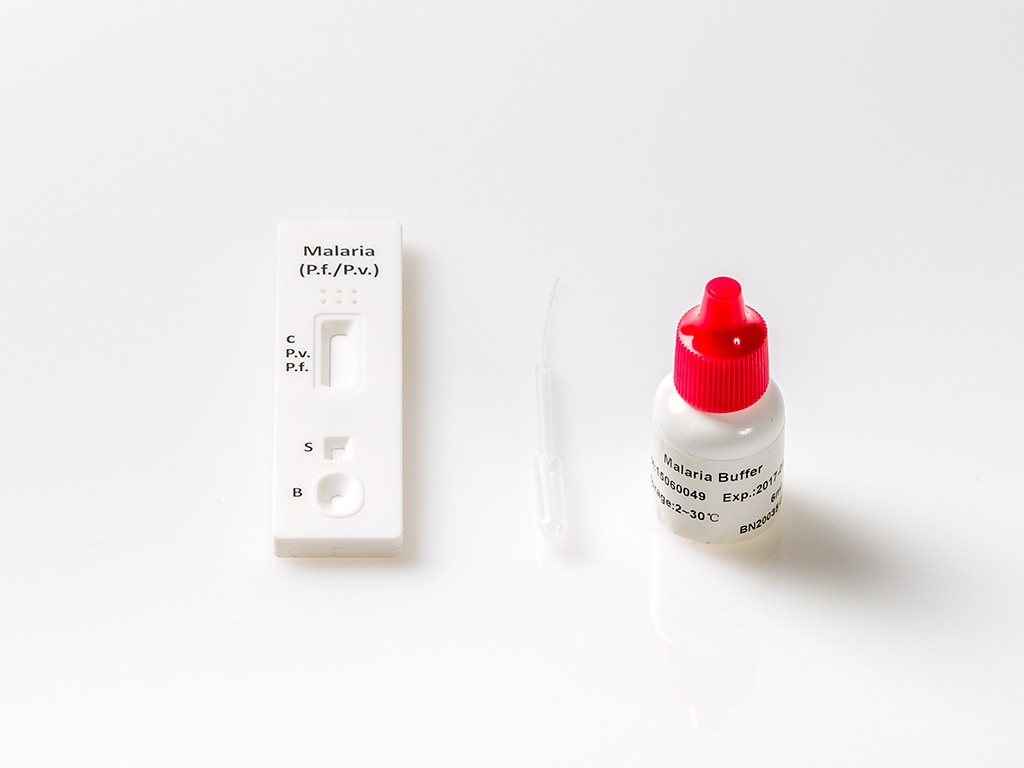
Malaria Rapid Test
A Malaria Test is a diagnostic test used to detect the presence of the malaria parasite in a person’s blood. These tests are crucial for diagnosing malaria, a potentially life-threatening mosquito-borne disease. They are offered by healthcare providers and laboratories, and they typically include rapid diagnostic tests (RDTs) and microscopic examination of blood smears. Rapid tests provide quick results, allowing for timely treatment and management of the disease. These tests are vital in regions where malaria is prevalent and for travelers to such areas.
Product information
The Malaria Rapid Test is a rapid qualitative assay for the detection of circulating antigens of P. falciparum (P.f.) and P. vivax (P.v.) in whole blood.
Advantages of Malaria test
- Easy to perform
- No complex sample collection needed
- Accurate test result
General information
Malaria is caused by a protozoan which invades human red blood cells.1 Malaria is one of the world’s most prevalent diseases. According to the WHO, the worldwide prevalence of the disease is estimated to be 300-500 million cases and over 1 million deaths each year. Most of these victims are infants, young children. Over half of the world’s population lives in malarious areas.
Microscopic analysis of appropriately stained thick and thin blood smears has been the standard diagnostic technique for identifying malaria infections for more than a century. The technique is capable of accurate and reliable diagnosis when performed by skilled microscopists using defined protocols. The skill of the microscopist and use of proven and defined procedures, frequently present the greatest obstacles to fully achieving the potential accuracy of microscopic diagnosis. Although there is a logistical burden associated with performing a time-intensive, labor-intensive, and equipment intensive procedure such as diagnostic microscopy, it is the training required to establish and sustain competent performance of microscopy that poses the greatest difficulty in employing this diagnostic technology.
The Malaria P.f./P.v. Rapid Test Cassette(Whole Blood) is a rapid test to qualitatively detect the presence of P. falciparum – specific HRP-II and P. vivax (P.v.). The test utilizes colloid gold conjugate to selectively detect P.f-specific and P. vivax (P.v.)-specific antigens in whole blood.
The Malaria P.f. /P.v. Rapid Test Cassette(Whole Blood) is a qualitative, membrane based immunoassay for the detection of P.f. and P.v. antigens in whole blood. The membrane is pre-coated with anti-HRP-II antibodies and anti-pLDH antibodies.
During testing, the whole blood specimen reacts with the dye conjugate, which has been pre-coated on the test cassette. The mixture then migrates upward on the membrane by capillary action, reacts with anti-Histidine-Rich Protein II (HRP-II) antibodies on the membrane on P.f. Test Line region and with anti-pLDH antibodies on the membrane on P.v. Line region. If the specimen contains HRP-II or Plasmodium-specific P. vivax LDH or both, a colored line will appear in P.f. line region or P.v. line region or two-colored lines will appear in P.f. line region and P.v. line region. The absence of the colored lines in P.f. line region or P.v. line region indicates that the specimen does not contain HRP-II and/or Plasmodium-specific P. vivax LDH. To serve as a procedure control, a colored line will always appear in the control line region indicating that proper volume of specimen has been added and membrane wicking has occurred.
Test procedure
- Allow the test, specimen, buffer and/or controls to reach room temperature (15-30°C) prior to testing.
- Bring the pouch to room temperature before opening it. Remove the test cassette from the sealed pouch and use it as soon as possible.
- Place the cassette on a clean and level surface.
- For Whole Blood specimen: Use a pipette: To transfer 5μL of whole blood to the specimen well, then add 3 drops of buffer (approximately 180μL).
- Use a disposal specimen dropper: Hold the dropper vertically, draw the specimen up to the Fill Line as shown in illustration below (approximately 5μL). Transfer the specimen to the specimen well, then add 3 drops of buffer (approximately 180μL), and start the timer.
- Wait for the colored line(s) to appear. Read results at 10 minutes. Do not interpret the result after 20 minutes.
Interpretation of the results
Negative result: Only one colored line appears in the control region (C).
Positive result: Two or Three distinct colored lines appear.
- falciparum or mixed malaria infection: one line appears in the control region; one line appears in P.v. line region and one line appears in P.f. line region.
- falciparum infection: one line appears in the control region, and one line appears in P.f .line region.
Non-falciparum Plasmodium species infection: one line appears in the control region and one line appears in P.v. line region.
*NOTE: The color intensity of P.f. or P.v. test lines may vary depending on the concentration of antigens, viz., HRP-II or P. vivax LDH present in the specimen.
Invalid result: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test device. If the problem persists, discontinue using the test kit immediately and contact your supplier.
| Product name | Malaria Rapid Test |
|---|---|
| Detection | P. falciparum (P.f.) and P. vivax (P.v.) Antigens in Whole Blood |
| Type | |
| Sample Type | |
| Pack Size | |
| Format | |
| Analyte Detection |
Related products
-
Medical Tests
Syphilis Rapid Test
Price requestA syphilis test is a medical examination that detects the presence of the bacterium Treponema pallidum, which causes syphilis, a sexually transmitted infection. This test is important for early diagnosis and treatment, as syphilis can lead to serious health issues if left untreated, including organ damage, neurological problems, and transmission to sexual partners or newborns. Early detection is crucial for effective management.
-
Heart Markers
HS CRP Rapid Test
Price requestThe high-sensitivity C-reactive protein (hs-CRP) test is a commercially available blood test used to measure the levels of C-reactive protein in the bloodstream. This test is highly sensitive and can detect even low levels of CRP, which is a marker of inflammation in the body. It is often used to assess an individual’s risk of cardiovascular disease and to monitor chronic inflammatory conditions. Commercially, this test is offered by various diagnostic laboratories and healthcare providers and is an essential tool for assessing an individual’s risk of heart disease and guiding preventive measures.
-
Medical Tests
PSA Rapid Test
Price requestA PSA (Prostate-Specific Antigen) test is a medical screening tool primarily used for the early detection of prostate cancer. This blood test measures the level of PSA, a protein produced by the prostate gland. Elevated PSA levels can be an indicator of prostate issues, including cancer. Regular PSA testing is recommended for men, particularly as they age, to help in the early diagnosis and management of prostate cancer.
-
Medical Tests
Vitamin D Rapid Test
Price requestA vitamin D test is a blood test that measures the levels of vitamin D in the bloodstream. This test is used to assess an individual’s vitamin D status, which is essential for bone health, immune function, and overall well-being. The test helps to diagnose vitamin D deficiencies or excesses and guide appropriate supplementation or treatment. It’s a valuable tool in maintaining optimal health.

 Drug Test
Drug Test Heart Markers
Heart Markers Hormone Tests
Hormone Tests Medical Tests
Medical Tests Microbiology
Microbiology Parasite Infection
Parasite Infection Proteins and Inflammatory Markers
Proteins and Inflammatory Markers Qualitative Controls
Qualitative Controls Tumor Marker
Tumor Marker Viruses
Viruses