Categories
Chlamydia Rapid Test
The Chlamydia Test is a diagnostic test used to detect the presence of Chlamydia trachomatis, a common sexually transmitted infection (STI). This test is typically performed on urine or swab samples from the genital area. It is a critical tool for diagnosing chlamydia infections, which can lead to various health complications if left untreated. Timely testing and detection help individuals receive appropriate treatment and prevent the spread of the infection.
Product information
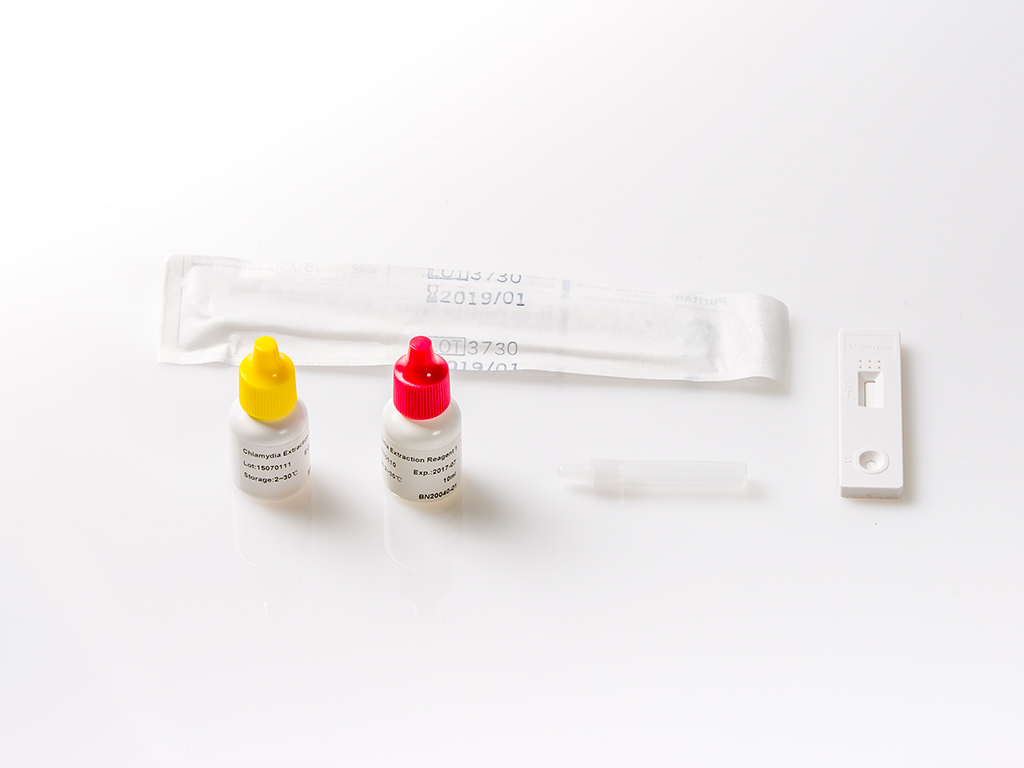
The Chlamydia Rapid Test a sensitive, specific and rapid test for the direct Chlamydia detection from endocervical or urethral swabs specimens, either from men or women, which is suitable for the physician use or for running
Advantages of Chlamydia test
- Easy to perform
- No complex sample collection needed
- Accurate test result
General information
The Chlamydia Rapid Test detects the chlamydia genus specific lipopolysaccharide (LPS) antigen. The method employs a unique combination of monoclonal-dye conjugate and solid phase antibodies to identify the LPS antigen in the swab samples with a high specificity and sensitivity.
In this test, the specimen (endocervical or urethral swab) is first treated with an extraction buffer to isolate Chlamydia LPS antigen when present and the extracted sample is then added in the device sample well (▷).
As the extract flows through the absorbent device, the labelled antibody-dye conjugate binds to the LPS antigen, forming an antibody antigen complex. This complex binds to the antibody in the positive reaction zone (T) and produces a pink-rose color band, when the LPS antigen concentration is present in the sample.
In the absence of LPS antigen, there is no line in the positive reaction zone. The reaction mixture continues flowing through the absorbent device, past the positive reaction zone and control zone. Unbound conjugate binds to the reagents in the control zone (C), producing a pink-rose color band, demonstrating that the reagents are functioning correctly.
Test procedure
- Remove The Chlamydia Rapid Test unit from its protective wrapper and place on a level surface.
- Label device with patient’s name or control number.
- Cap the extraction tube with the filter dropper (provided) and apply four (4) drops (150 μL) of extract to the sample well (▷) of the device.
- Allow each drop to absorb before adding the next one and avoid bubbles in the sample window when adding liquids.
- Let the reaction proceed and read the result at 20 minutes after addition of the extract into the sample well (▷).
Interpretation of the results
Negative result: Only one pink colored line appears in the control zone (C) showing that the test has been carried out correctly. No line in the test zone (T). The test is negative for chlamydial antigen.
Positive result: In addition to the colored line in control zone (C), a clearly-distinguishable pink colored line also appears in the test zone (T), indicating a positive result for chlamydia antigen in the sample. Even a weak line in the test zone (T) should be interpreted as a positive result. Different intensities between test (T) and control (C) may occur but do not affect the interpretation of the results.
Invalid result: If no line appears in the control zone (C), the test must be repeated with another test unit. Either a fresh specimen may be collected or the remaining extraction mixture can be used for this purpose.
| Product name | Chlamydia Rapid Test |
|---|---|
| Detection | Chlamydia Genus Specific Lipopolysaccharide (LPS) antigen |
| Type | |
| Sample Type | |
| Pack Size | |
| Format | |
| Analyte Detection |
Related products
-
Heart Markers
D-Dimer Rapid Test
Price requestThe D-dimer test is a test that measures the levels of D-dimer, a protein fragment, in the bloodstream. It’s a critical diagnostic tool for detecting abnormal blood clot formation, often associated with conditions like deep vein thrombosis and pulmonary embolism. Healthcare providers use this test to assess the likelihood of clot-related disorders, aiding in diagnosis and guiding treatment decisions. It plays a crucial role in ensuring patient safety and timely medical interventions.
-
Medical Tests
Syphilis Rapid Test
Price requestA syphilis test is a medical examination that detects the presence of the bacterium Treponema pallidum, which causes syphilis, a sexually transmitted infection. This test is important for early diagnosis and treatment, as syphilis can lead to serious health issues if left untreated, including organ damage, neurological problems, and transmission to sexual partners or newborns. Early detection is crucial for effective management.
-
Medical Tests
Malaria Rapid Test
Price requestA Malaria Test is a diagnostic test used to detect the presence of the malaria parasite in a person’s blood. These tests are crucial for diagnosing malaria, a potentially life-threatening mosquito-borne disease. They are offered by healthcare providers and laboratories, and they typically include rapid diagnostic tests (RDTs) and microscopic examination of blood smears. Rapid tests provide quick results, allowing for timely treatment and management of the disease. These tests are vital in regions where malaria is prevalent and for travelers to such areas.

 Drug Test
Drug Test Heart Markers
Heart Markers Hormone Tests
Hormone Tests Medical Tests
Medical Tests Microbiology
Microbiology Parasite Infection
Parasite Infection Proteins and Inflammatory Markers
Proteins and Inflammatory Markers Qualitative Controls
Qualitative Controls Tumor Marker
Tumor Marker Viruses
Viruses