Categories
Clostridium Difficile Toxin A/B Rapid Test SC
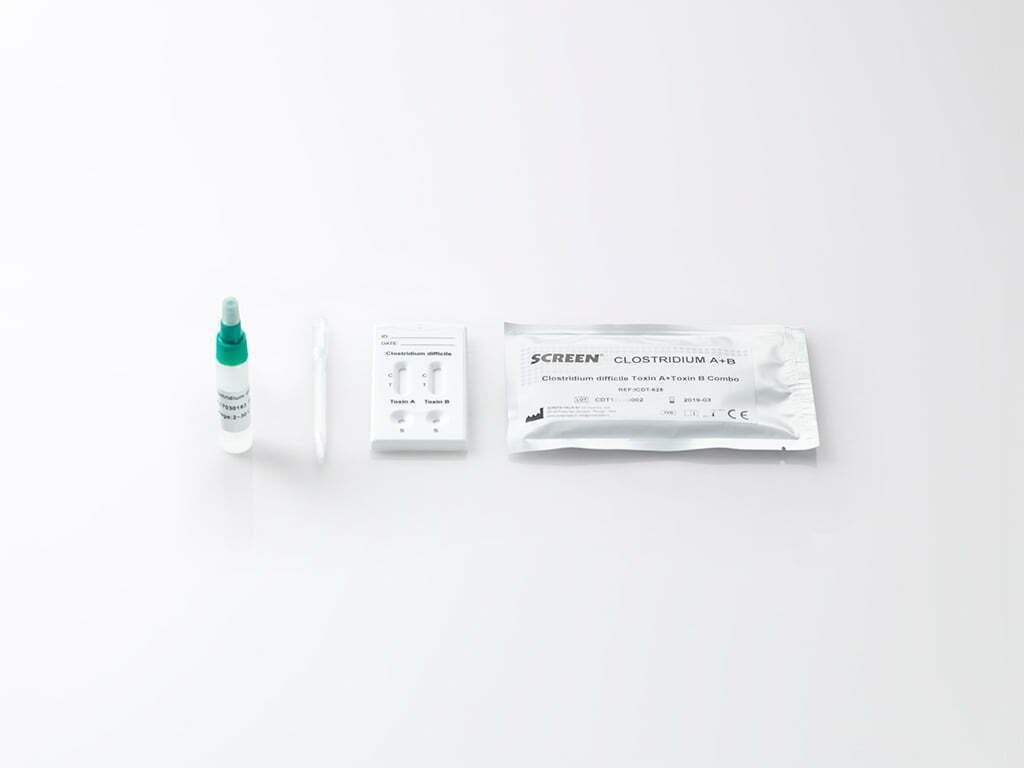
The Clostridium difficile Toxin A+ Toxin B Combo Rapid Test Cassette (Feces) is a rapid chromatographic immunoassay for the qualitative detection of Clostridium difficile Toxin A and Toxin B antigens in the human feces specimen.
Product information
Clostridium Difficile Toxin A and Toxin B Rapid Test is a lateral flow, immunochromatographic rapid test for the qualitative detection of Clostridium difficile Toxin A and Toxin B in human feces.
Advantages of Clostridium difficile Toxin A/B test
- Easy to perform
- No complex sample collection needed
- Accurate test result
General information
Clostridium difficile is an anaerobic bacteria acting as an opportunistic pathogen: it grows in the intestine when the normal flora has been altered by treatment with antibiotics. Toxinogenic strains of Clostridium difficile cause infections from mild-diarrhea to pseudomembranous colitis, potentially leading to death. Disease is caused by two toxins produced by toxinogenic strains of C.difficile: Toxin A (tissue-damaging enterotoxin) and Toxin B (cytotoxin). Some strains produce both toxins A and B, some others produce Toxin B only. The potential role of a third (binary) toxin in pathogenicity is still debated. Clostridium difficileToxinA+Toxin B Combo Rapid Test Cassette(Feces) detects two distinct antigens in fecal specimens for C. difficile, viz., Toxin A and Toxin B on two different test strips in a single test cassette, thus simultaneously detecting two antigens specific to Clostridium difficile.
For C.difficile-specific Toxin A
Testing The membrane is precoated with anti-C.diff Toxin A antibody and anti-C.diff Toxin A antibody on the test line region. During testing, the specimen reacts with the particle coated with anti-C.diff Toxin A antibody. The mixture migrates upward on the membrane chromatographically by capillary action to react with anti-C.diff Toxin A antibody on the membrane and generate a colored line. The presence of this colored line in the test line region indicates a positive result, while its absence indicates a negative result. To serve as a procedural control, a colored line will always appear in the control line region, indicating that the proper volume of specimen has been added and membrane wicking has occurred.
For C.difficile-specifc Toxin B
Testing The membrane is precoated with anti-C.diff Toxin B antibody and anti-C.diff Toxin B antibody on the test line region. During testing, the specimen reacts with the particle coated with anti-C.diff Toxin B antibody. The mixture migrates upward on the membrane chromatographically by capillary action to react with anti-C.diff Toxin B antibody on the membrane and generate a colored line. The presence of this colored line in the test line region indicates a positive result, while its absence indicates a negative result. To serve
as a procedural control, a colored line will always appear in the control line region, indicating that the proper volume of specimen has been added and membrane wicking has occurred.
Specimen Collection
The stool specimens must be tested as soon as possible after collection. If necessary, original feces specimen may be stored at 2-8°C for 3 days or -20°C for longer periods of time; extracted specimen in buffer may be stored at 2-8°C for 1 week or -20°C for
longer periods of time. Make sure that the specimens are not treated with solutions containing formaldehyde or its derivatives.
Test Procedure
Allow the test, specimen, stool collection buffer and/or control to equilibrate to room temperature (15-30°C) prior to testing
1. To collect fecal specimens:
Collect sufficient quantity of feces (1-2mL or 1-2g) in a clean, dry specimen collection container to obtain enough antigens (if present).Best results will be obtained if the assay is performed within 6 hours after collection. Specimen collected may be stored for 3 days at 2-8°C if not tested within 6 hours. For long term storage, specimens should be kept below -20°C
2. To process fecal specimens
For Solid Specimens: Unscrew the cap of the specimen collection tube, then randomly stab the specimen collection applicator into the fecal specimen at least 3 different sites to collect approximately 50 mg of feces (equivalent to 1/4 of a pea). Do not scoop the fecal specimen.
For Liquid Specimens: Hold the dropper vertically, aspirate fecal specimens, and then transfer 2 drops of the liquid specimen (approximately 80 µL) into the specimen collection tube containing the extraction buffer.
3. Tighten the cap onto the specimen collection tube, then shake the specimen collection tube vigorously to mix the specimen and the extraction buffer. Leave the collection tube for reaction for 2 minutes. Bring the pouch to room temperature before opening it. Remove the test cassette from the foil pouch and use it as soon as possible. Best results will be obtained if the test is performed immediately after opening the foil pouch.
4. Hold the specimen collection tube upright and unscrew the tip of the specimen collection tube. Invert the specimen collection tube and transfer 3 full drops of the extracted specimen (approximately 120L) to the specimen well (S) of the test
cassette, then start the timer. Avoid trapping air bubbles in the specimen well (S).
5. Read the results at 10 minutes after dispensing the specimen. Do not read results after 20 minutes.
Note: If the specimen does not migrate (presence of particles), centrifuge the diluted sample contained in the extraction buffer vial. Collect 120µL of supernatant, dispense into the specimen well (S). Start the timer and continue from step 5 onwards in the
above instructions for use.
Interpretation of the results
The test results appear in two different test windows respectively for Toxin A or Toxin B. The interpretation criteria remain the same for positivity or negativity for specific antigens under tests as per indication of the respective Test window. The results are to be interpreted as follows:
POSITIVE: Two distinct colored lines appear. One colored line should be in the control line region (C) and another apparent colored line should be in the test line region (T).
*NOTE: The intensity of the color in the test line region (T) will vary depending on the concentration of Clostridium difficile antigen present in the specimen. Therefore, any shade of color in the test line region (T) should be considered positive.
NEGATIVE: One colored line appears in the control line region (C). No line appears in the test line region (T).
INVALID: Control line (C) fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test cassette. If the problem persists,
discontinue using the test kit immediately and contact your local supplier.
| Product name | Clostridium Difficile Toxin A/B Rapid Test SC |
|---|---|
| Detection | Clostridium Difficile Toxin A and Toxin B |
| Type | |
| Sample Type | |
| Pack Size | |
| Format | |
| Analyte Detection |
Related products
-
Medical Tests
Chlamydia Rapid Test Swab/Urine
Price requestThe Chlamydia Rapid Test Cassette is a rapid chromatographic immunoassay for the qualitative detection of Chlamydia trachomatis in female cervical swab, male urethral swab and male urine specimens to aid in the diagnosis of Chlamydia infection.
-
Medical Tests
RSV Rapid Test
Price requestThe Respiratory Syncytial Virus (RSV) test is used to detect the presence of RSV in respiratory secretions, typically collected via a nasal swab or throat swab. RSV is a common virus that causes respiratory infections, especially in infants and young children. This test is to confirm RSV infections, enabling appropriate treatment and care for affected individuals, particularly during the RSV season in colder months.
-
Heart Markers
BNP Rapid Test
Price requestThe B-type natriuretic peptide (BNP) test is a blood test used to measure the levels of BNP in the bloodstream. BNP is a hormone released by the heart in response to increased pressure or stress, often associated with heart failure. This test is used to diagnose and monitor heart failure and other cardiac conditions. It allows to assess cardiac function, guide treatment, and monitor the effectiveness of heart failure therapy. Elevated BNP levels can indicate heart problems and help physicians make informed decisions about patient care.
-
Heart Markers
D-Dimer Rapid Test
Price requestThe D-dimer test is a test that measures the levels of D-dimer, a protein fragment, in the bloodstream. It’s a critical diagnostic tool for detecting abnormal blood clot formation, often associated with conditions like deep vein thrombosis and pulmonary embolism. Healthcare providers use this test to assess the likelihood of clot-related disorders, aiding in diagnosis and guiding treatment decisions. It plays a crucial role in ensuring patient safety and timely medical interventions.

 Drug Test
Drug Test Heart Markers
Heart Markers Hormone Tests
Hormone Tests Medical Tests
Medical Tests Microbiology
Microbiology Parasite Infection
Parasite Infection Proteins and Inflammatory Markers
Proteins and Inflammatory Markers Qualitative Controls
Qualitative Controls Tumor Marker
Tumor Marker Viruses
Viruses