Categories

FOB Rapid Test
The Fecal Occult Blood (FOB) test, as a stool test, is used to detect the presence of hidden or occult blood in the feces. This test is vital for identifying gastrointestinal bleeding and potential issues such as colorectal cancer, polyps, or other digestive disorders. The test enables the early detection of colorectal health problems, which can lead to timely medical intervention and improved outcomes.
Product information
The FOB Rapid Test is a rapid qualitative, immunochromatographic assay for the detection of fecal occult blood in colorectal.
Advantages of FOB test
- Easy to perform
- No complex sample collection needed
- Accurate test result
General information
Colorectal cancer has an annual incidence worldwide of more than 600,000 cases and ranks as the third most common cancer. As with all cancers, early detection of lesions increases considerably the survival rate of the patient. Among people over 45 years old, 10% have colorectal polyps of which 1% will become malignant. Relying on the fact that many polyps larger than 0.5 cm can bleed, fecal occult blood testing appears to be a simple and cheap screening method for colorectal cancer when compared to colonoscopy.
For many years, chemical techniques based on the pseudoperoxidase activity of hemoglobin were used with the drawback of low sensitivity and poor specificity. Immunological methods with improved sensitivity and specificity for human blood are starting to be used in spite of their greater technical complexity when compared to guaiac tests.
FOB Rapid Test is a rapid qualitative, immunochromatographic assay for the detection of fecal occult blood in colorectal cancer. The method employs a unique combination of monoclonal dye conjugate and polyclonal solid phase antibodies to selectively identify human hemoglobin with a high degree of sensitivity and specificity.
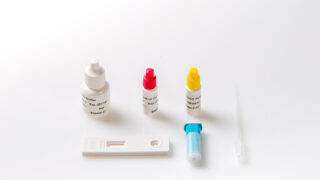
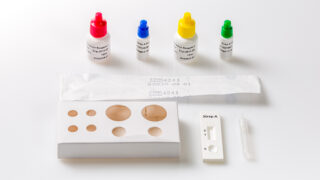
After collection in a special collection device containing extraction solution, the sample is dissolved and a few drops of this extract are added to the sample well of the reaction device. As the test sample flows through the absorbent device, the labelled antibody-dye conjugate binds to the hemoglobin antigen forming an antibody antigen complex.
This complex binds to the hemoglobin antibody in the test zone (T) producing a pink-rose colored band. In the absence of hemoglobin, there is no line in the test zone (T). The reaction mixture continues to flow through the absorbent device past. Unbound conjugate binds to the reagents in the control zone (C), producing a pink-rose colored band, demonstrating that the reagents are functioning correctly.
Test procedure
- Allow the samples and reagents to return to room temperature prior to testing
- Remove the test device from the pouch.
- Break the tip of the collection device, squeeze 4 drops (100 μL) of extracted sample into the sample well (▷) of the reaction device.
- Read the results of the test 10 minutes after adding sample on the device.
Interpretation of the results
Negative result: Only one colored band appears on the control zone (C).
Positive result: Two clearly distinguishable lines appear (T and C).
Invalid result: There is no distinct colored band in the control zone (C) the test is inconclusive. It is recommended, in this case, to repeat the test.
| Product name | FOB Rapid Test |
|---|---|
| Detection | Fecal Occult Blood |
| Type | |
| Sample Type | |
| Pack Size | |
| Format | |
| Analyte Detection |
Related products
-
Heart Markers
CK-MB Rapid Test
Price requestThe CK-MB test, or Creatine Kinase-MB test, is a commercially available diagnostic blood test used to measure the levels of a specific enzyme called creatine kinase-MB in the bloodstream. This test is primarily used to assess and diagnose heart muscle damage, such as during a heart attack.
Elevated CK-MB levels in the blood can indicate myocardial injury, as this enzyme is primarily found in the heart muscle. It is an important tool for healthcare professionals to help confirm or rule out acute coronary syndrome and other cardiac conditions.
-
Medical Tests
Mononucleosis Rapid Test
Price requestMononucleosis, known as the “mono spot” test or the infectious mononucleosis test, is a diagnostic test that helps identify the presence of the Epstein-Barr virus (EBV) in a person’s blood. EBV is the virus responsible for causing mononucleosis, a common viral infection often referred to as the “kissing disease.” The test is used to confirm a mononucleosis diagnosis. It plays a crucial role in identifying the underlying cause of symptoms like fatigue, fever, and sore throat, enabling appropriate medical care and management.
-
Medical Tests
Strep A Rapid Test
Price requestThe Streptococcus A test, known as a rapid strep test, is a diagnostic test used to detect the presence of group A Streptococcus bacteria (Streptococcus pyogenes) in the throat. It is essential for diagnosing strep throat, a common bacterial infection, and helps in quickly identifying the causative agent of symptoms like sore throat and fever. Rapid results from this test allow for timely treatment with antibiotics, reducing the severity and duration of the illness.
-
Medical Tests
Legionella Pneumophila Rapid Test
Price requestThe Legionella pneumophila test is a diagnostic test that specifically detects the Legionella pneumophila bacteria, a common cause of Legionnaires’ disease. This test is used to assess the presence of this pathogen in water sources, such as cooling towers and plumbing systems. It’s crucial for ensuring the safety of water systems in environments like hospitals, hotels, and industrial facilities.

 Drug Test
Drug Test Heart Markers
Heart Markers Hormone Tests
Hormone Tests Medical Tests
Medical Tests Microbiology
Microbiology Parasite Infection
Parasite Infection Proteins and Inflammatory Markers
Proteins and Inflammatory Markers Qualitative Controls
Qualitative Controls Tumor Marker
Tumor Marker Viruses
Viruses