Categories
FOB Rapid Test SC
The Fecal Occult Blood (FOB) test, as a stool test, is used to detect the presence of hidden or occult blood in the feces. This test is vital for identifying gastrointestinal bleeding and potential issues such as colorectal cancer, polyps, or other digestive disorders. The test enables the early detection of colorectal health problems, which can lead to timely medical intervention and improved outcomes.
Product information
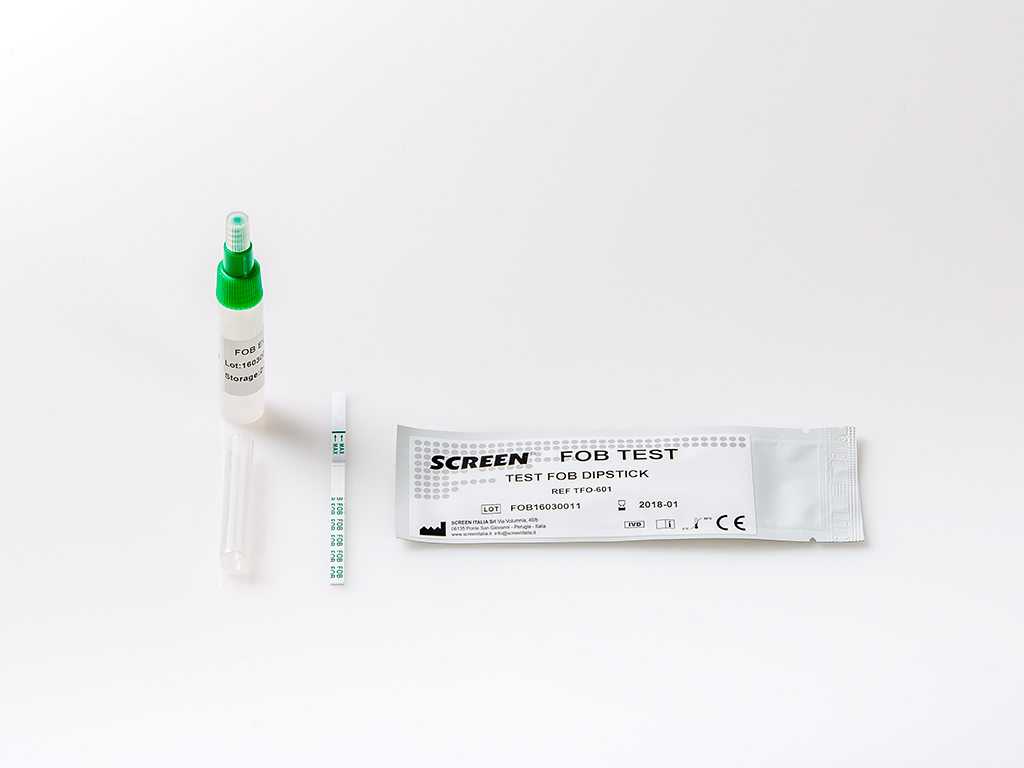
The FOB Rapid Test is a rapid chromatographic immunoassay for the qualitative detection of Human Occult Blood in feces.
Advantages of FOB test
- Easy to perform
- No complex sample collection needed
- Accurate test result
General information
Many diseases can cause hidden blood in the feces. This is also known as Fecal Occult Blood (FOB), Human Occult Blood, or Human Hemoglobin. In the early stages, gastrointestinal problems such as colon cancer, ulcers, polyps, colitis, diverticulitis, and
fissures may not show any visible symptoms, only occult blood. Traditional guaiac-based methods lack sensitivity and specificity, and also have diet restrictions prior to testing. The FOB Rapid Test Cassette (Feces) is a rapid test to qualitatively detect low levels of Fecal Occult Blood. The test uses a double antibody sandwich assay to selectively detect Fecal Occult Blood at 50ng/ml or higher, or 6μg/g feces. In addition, unlike guaiac assays, the accuracy of the test is not affected by the diet of the patients.
The FOB Rapid Test Cassette (Feces) is a qualitative, lateral flow immunoassay for the detection of Human Occult Blood in feces. The membrane is precoated with anti-hemoglobin antibody on the test line region of the test. During testing, the specimen
reacts with the particle coated with anti-hemoglobin antibody. The mixture migrates upward on the membrane chromatographically by capillary action to react with anti-hemoglobin antibody on the membrane and generate a colored line. The presence of this
colored line in the test line region indicates a positive result, while its absence indicates a negative result. To serve as a procedural control, a colored line will always appear in the control line region, indicating that the proper volume of specimen has been added and membrane wicking has occurred.
Specimen Collection
- Specimens should not be collected during or within three days of a menstrual period, or if the patient suffers from bleeding hemorrhoids or blood in the urine.
- Alcohol, aspirin and other medications taken in excess may cause gastrointestinal irritation resulting in occult bleeding. Such substances should be discontinued at least 48 hours prior to testing.
- No dietary restrictions are necessary before using the FOB Rapid Test Cassette.
Test procedure
Allow the test, specimen, buffer and/or controls to reach room temperature (1530°C) prior to testing.
1. To collect fecal specimens:
Collect sufficient quantity of feces (1-2 mL or 1-2 g) in a clean, dry specimen collection container to obtain maximum antigens (if present). Best results will be obtained if the assay is performed within 6 hours after collection. Specimen collected may be stored
for 3 days at 2-8℃ if not tested within 6 hours. For long term storage, specimens should be kept below -20℃.
2. To process fecal specimens:
• For Solid Specimens: Unscrew the cap of the specimen collection tube, then randomly stab the specimen collection applicator into the fecal specimen in at least 3 different sites to collect approximately 50 mg of feces (equivalent to 1/4 of a pea). Do not scoop the fecal specimen.
• For Liquid Specimens: Hold the dropper vertically, aspirate fecal specimens, and then transfer 2 drops (approximately 80 μL) into the specimen collection tube containing the extraction buffer.
3. Tighten the cap onto the specimen collection tube, then shake the specimen collection tube vigorously to mix the specimen and the extraction buffer. Leave the tube alone for 2 minutes.
4. Bring the pouch to room temperature before opening it. Remove the test cassette from the foil pouch and use it within one hour. Best results will be obtained if the test is performed immediately after opening the foil pouch.
5. Hold the specimen collection tube upright and open the cap onto the specimen collection tube. Invert the specimen collection tube and transfer 2 full drops of the extracted specimen (approximately 80 μL) to the specimen well (S) of the test cassette, then start the timer. Avoid trapping air bubbles in the specimen well (S).
6. Read results at 5 minutes after dispensing the specimen. Do not read results after 10 minutes.
7. Note: If the specimen does not migrate (presence of particles), centrifuge the extracted specimens contained in the extraction buffer vial. Collect 80 μL of supernatant, dispense into the specimen well (S) of a new test cassette and start afresh following the
instructions mentioned above.
Interpretation of the results
POSITIVE: Two colored lines appear. One colored line should be in the control line region (C) and another apparent colored line should be in the test line region (T).
*NOTE: The intensity of the color in the test line region (T) will vary depending on the concentration of Fecal Occult Blood present in the specimen. Therefore, any shade of color in the test line region (T) should be considered positive.
NEGATIVE: One colored line appears in the control line region (C). No line appears in the test line region (T).
INVALID: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test. If the problem persists, discontinue using
the test kit immediately and contact your local supplier.
| Product name | FOB Rapid Test |
|---|---|
| Detection | Fecal Occult Blood |
| Type | |
| Sample Type | |
| Pack Size | |
| Format | |
| Analyte Detection |
Related products
-
Heart Markers
HS CRP Rapid Test
Price requestThe high-sensitivity C-reactive protein (hs-CRP) test is a commercially available blood test used to measure the levels of C-reactive protein in the bloodstream. This test is highly sensitive and can detect even low levels of CRP, which is a marker of inflammation in the body. It is often used to assess an individual’s risk of cardiovascular disease and to monitor chronic inflammatory conditions. Commercially, this test is offered by various diagnostic laboratories and healthcare providers and is an essential tool for assessing an individual’s risk of heart disease and guiding preventive measures.
-
Medical Tests
CA-125 Rapid Test
Price requestCA-125 is a protein marker that can be measured rapidly with serum, plasma and whole blood. It is primarily used as a tumor marker, specifically for ovarian cancer. Elevated CA-125 levels can indicate the presence of ovarian cancer, but it can also be elevated in other conditions, such as endometriosis and certain noncancerous conditions. The CA-125 test is a valuable tool for healthcare professionals in screening, diagnosing, and monitoring ovarian cancer and other gynecological disorders.
-
Medical Tests
RSV Rapid Test
Price requestThe Respiratory Syncytial Virus (RSV) test is used to detect the presence of RSV in respiratory secretions, typically collected via a nasal swab or throat swab. RSV is a common virus that causes respiratory infections, especially in infants and young children. This test is to confirm RSV infections, enabling appropriate treatment and care for affected individuals, particularly during the RSV season in colder months.
-
Medical Tests
FOB Rapid Test
Price requestThe Fecal Occult Blood (FOB) test, as a stool test, is used to detect the presence of hidden or occult blood in the feces. This test is vital for identifying gastrointestinal bleeding and potential issues such as colorectal cancer, polyps, or other digestive disorders. The test enables the early detection of colorectal health problems, which can lead to timely medical intervention and improved outcomes.

 Drug Test
Drug Test Heart Markers
Heart Markers Hormone Tests
Hormone Tests Medical Tests
Medical Tests Microbiology
Microbiology Parasite Infection
Parasite Infection Proteins and Inflammatory Markers
Proteins and Inflammatory Markers Qualitative Controls
Qualitative Controls Tumor Marker
Tumor Marker Viruses
Viruses