Categories
H. Pylori Antigen Rapid Test SC
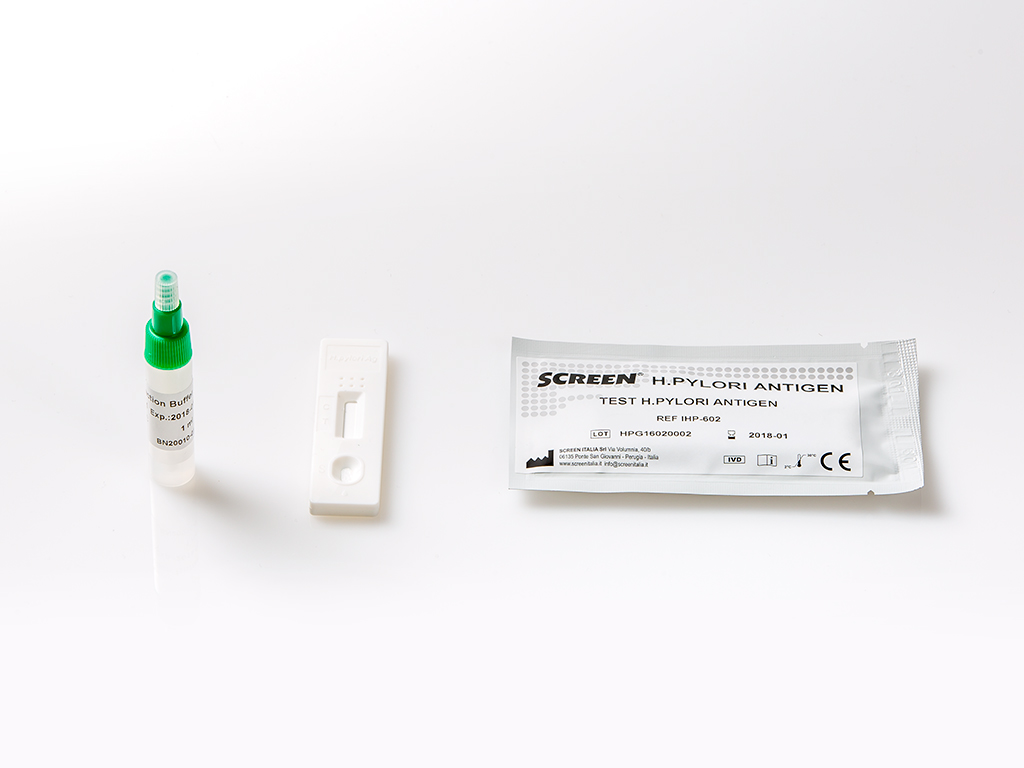
The H. Pylori Antigen Rapid Test Cassette (Feces) is a rapid chromatographic immunoassay for the qualitative detection of H. pylori antigens in human feces specimens to aid in the diagnosis of H. pylori infection.
Product information
The H. pylori Antigen Rapid Test Cassette (Feces) is a rapid chromatographic immunoassay for the qualitative detection of H. pylori antigens in human feces specimens to aid in the diagnosis of H. pylori infection.
Advantages of H. Pylori Ag test
- Easy to perform
- No complex sample collection needed
- Accurate test result
General information
H. pylori is a small, spiral-shaped bacterium that lives in the surface of the stomach and duodenum. It is implicated in the etiology of a variety of gastrointestinal diseases, including duodenal and gastric ulcer, non-ulcer dyspepsia and active and chronic gastritis. Both invasive and non-invasive methods are used to diagnose H. pylori infection in patients with symptoms of gastrointestinal disease. Specimen-dependent and costly invasive diagnostic methods include gastric or duodenal biopsy followed by urease testing (presumptive), culture, and/or histologic staining.
A very common approach to the diagnosis of H. pylori infection is the serological identification of specific antibodies in infected patients. The main limitation of serology test is the inability to distinguish current and past infections. Antibody may be present in the patient’s serum long after eradication of the organisms. HpSA (H. pylori Stool Antigen) testing is gaining popularity for diagnosis of H. pylori infection and also for monitoring the efficacy of the treatment of H. pylori infection. Studies have found that more than 90% of patients with duodenal ulcer and 80% of patients with gastric ulcer are infected with H. pylori. The H. pylori Antigen Rapid Test Cassette (Feces) is a rapid chromatographic immunoassay for the qualitative detection of H. pylori antigens in human feces specimens, providing results in 10 minutes. The test utilizes antibodies specific for H. pylori antigens to selectively detect H. pylori antigens in human feces specimens.
The H. pylori Antigen Rapid Test Cassette (Feces) is a qualitative, lateral flow immunoassay for the detection of H. pylori antigens in human feces specimens. In this test, the membrane is pre-coated with anti-H. pylori antibodies on the test line region of the test. During testing, the specimen reacts with the particle coated with anti-H. pylori antibodies. The mixture migrates upward on the membrane by capillary action to react with anti-H. pylori antibodies on the membrane and generate a colored line. The presence of this colored line in the test region indicates a positive result, while its absence indicates a negative result. To serve as a procedural control, a colored line will always appear in the control line region indicating that proper volume of specimen has been added and membrane wicking has occurred.
Specimen Collection
- The feces specimen must be collected in clean, dry, waterproof container containing no detergents, preservatives or transport media.
- Bring the necessary reagents to room temperature before use.
- If specimens are to be shipped, they should be packed in compliance with federal regulations covering the transportation of etiologic agents.
Test Procedure
Allow the test cassette, specimen, buffer and/or controls to reach room temperature (15-30°C) prior to testing.
1. To collect fecal specimens:
Collect sufficient quantity of feces (1-2 mL or 1-2 g) in a clean, dry specimen collection container to obtain maximum antigens (if present). Best results will be obtained if the assay is performed within 6 hours after collection. Specimen collected may be stored for 3 days at 2-8℃ if not tested within 6 hours. For long term storage, specimens should be kept below -20℃.
2. To process fecal specimens:
For Solid Specimens: Unscrew the cap of the specimen collection tube, then randomly stab the specimen collection applicator into the fecal specimen in at least 3 different sites to collect approximately 50 mg of feces (equivalent to 1/4 of a pea). Do not scoop the fecal specimen.
For Liquid Specimens: Hold the dropper vertically, aspirate fecal specimens, and then transfer approximately 80 μL into the specimen collection tube containing the extraction buffer. Tighten the cap onto the specimen collection tube, then shake the specimen collection tube vigorously to mix the specimen and the extraction buffer. Leave the tube alone for 2 minutes.
3. Bring the pouch to room temperature before opening it. Remove the test cassette from the foil pouch and use it within one hour. Best results will be obtained if the test is performed immediately after opening the foil pouch.
4. Hold the specimen collection tube upright and open the cap onto the specimen collection tube. Invert the specimen collection tube and transfer 2 full drops of the extracted specimen (approximately 80 μL) to the specimen well (S) of the test cassette, then
start the timer. Avoid trapping air bubbles in the specimen well (S). See illustration below.
5. Read results at 10 minutes after dispensing the specimen. Do not read results after 20 minutes.
Note: If the specimen does not migrate (presence of particles), centrifuge the extracted specimens contained in the extraction buffer vial. Collect 80 μL of supernatant, dispense into the specimen well.
Interpretation of the results
POSITIVE: Two lines appear. One colored line should be in the control line region (C) and another apparent colored line should be in the test line region (T).
*NOTE: The intensity of the color in the test line region (T) will vary depending on the concentration of H.pylori antigen present in the specimen. Therefore, any shade of color in the test line region (T) should be considered positive.
NEGATIVE: One colored line appears in the control line region (C). No line appears in the test line region (T).
INVALID: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test. If the problem persists, discontinue using the
test kit immediately and contact your local distributor.
| Product name | H. Pylori Antigen Rapid Test SC |
|---|---|
| Detection | Helicobacter Pylori Antigen |
| Type | |
| Sample Type | |
| Pack Size | |
| Format | |
| Analyte Detection |
Related products
-
Hormone Tests
hCG 25mIU/mL Rapid Test
Price requestThe human chorionic gonadotropin (hCG) test is a diagnostic test used to detect the presence of hCG, a hormone produced during pregnancy. This test is typically performed on a serum or urine sample. It is used to confirm pregnancy, as hCG levels rise early in gestation.
-
Medical Tests
Syphilis Rapid Test
Price requestA syphilis test is a medical examination that detects the presence of the bacterium Treponema pallidum, which causes syphilis, a sexually transmitted infection. This test is important for early diagnosis and treatment, as syphilis can lead to serious health issues if left untreated, including organ damage, neurological problems, and transmission to sexual partners or newborns. Early detection is crucial for effective management.
-
Medical Tests
Chlamydia Rapid Test
Price requestThe Chlamydia Test is a diagnostic test used to detect the presence of Chlamydia trachomatis, a common sexually transmitted infection (STI). This test is typically performed on urine or swab samples from the genital area. It is a critical tool for diagnosing chlamydia infections, which can lead to various health complications if left untreated. Timely testing and detection help individuals receive appropriate treatment and prevent the spread of the infection.
-
Medical Tests
Mononucleosis Rapid Test
Price requestMononucleosis, known as the “mono spot” test or the infectious mononucleosis test, is a diagnostic test that helps identify the presence of the Epstein-Barr virus (EBV) in a person’s blood. EBV is the virus responsible for causing mononucleosis, a common viral infection often referred to as the “kissing disease.” The test is used to confirm a mononucleosis diagnosis. It plays a crucial role in identifying the underlying cause of symptoms like fatigue, fever, and sore throat, enabling appropriate medical care and management.

 Drug Test
Drug Test Heart Markers
Heart Markers Hormone Tests
Hormone Tests Medical Tests
Medical Tests Microbiology
Microbiology Parasite Infection
Parasite Infection Proteins and Inflammatory Markers
Proteins and Inflammatory Markers Qualitative Controls
Qualitative Controls Tumor Marker
Tumor Marker Viruses
Viruses